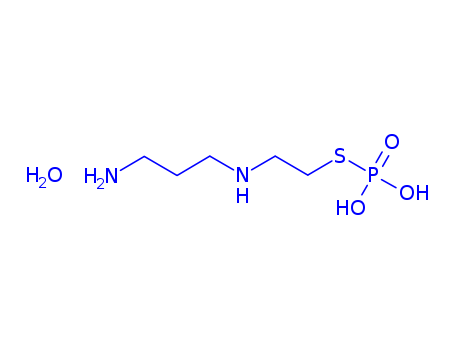
112901-68-5
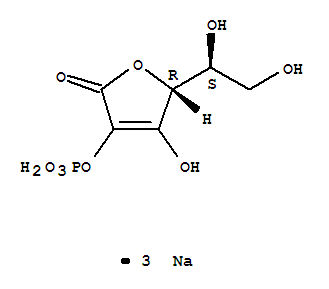
- Product Name:Amifostine trihydrate
- Molecular Formula:C5H21N2O6PS
- Purity:99%
- Molecular Weight:232.241
Product Details
Factory Supply Industrial Grade Amifostine trihydrate 112901-68-5 with Best Price
- Molecular Formula:C5H15N2O3PS*H2O
- Molecular Weight:232.241
- Vapor Pressure:4.9E-09mmHg at 25°C
- Boiling Point:441.7 °C at 760 mmHg
- PKA:pKa1 <2.0; pKa2 4.2; pKa3 9.0; pKa4 11.7(at 25℃)
- Flash Point:220.9 °C
- PSA:158.38000
- Density:1.367g/cm3
- LogP:0.64900
2-(3-Aminopropylamino)ethylsulfanylphosphonic acid trihydrate(Cas 112901-68-5) Usage
|
Biochem/physiol Actions |
Radioprotective agent. Selectively protects normal tissues from the damaging effects of anti-neoplastic radiation therapy. Selectivity is due to preferential uptake by normal tissues and subsequent metabolic activation to 2-(3-aminopropyl)aminoethanethiol. |
|
Synthesis |
1). In the 50L stainless steel reactor, add 13.6kg of pure water, 19.2mol (7.6kg) of sodium thiophosphate dodecahydrate, add N-(2-bromoethyl)-1,3-propanediamine bisulfite under stirring Hydrobromide salt 19.8mol (6.8kg, 3% excess), 11.0kg DMSO was added slowly while maintaining the reaction temperature not to exceed 25°C. After the dropwise addition of DMSO, the reaction solution was monitored with silver nitrate solution until no black precipitate was precipitated, and the reaction was completed. Centrifugal filtration to obtain 6.1 kg of crude amifostine trihydrate.2). In the 50L stainless steel crystallization kettle, add 22.0kg of pure water, add 6.1kg of the crude 3-aminopropylamine ethyl thiophosphoric acid prepared in the previous step, dissolve at room temperature, add 95.0g of activated carbon, and stir at room temperature for 15 minutes , filter, add methanol 8.7kg to the mother liquor, cool down to 0 ℃ through cooling water, maintain 18 hours, centrifugal filter, obtain 4.8kg of 3-aminopropylamine ethyl thiophosphoric acid without crystal water once recrystallization.3). Add 20.0kg of pure water to the 50L stainless steel crystallization kettle, add 4.8kg of 3-aminopropylamine ethyl thiophosphoric acid without crystal water obtained by the first recrystallization, stir and dissolve at room temperature, add 75.0g of activated carbon , stirred at room temperature for 15 minutes, filtered, added 3.2kg of ethanol to the mother liquor, cooled to 0°C through cooling water, maintained for 18 hours, centrifugally filtered, and vacuum dried at 30°C to obtain 3.6kg of amifostine trihydrate. |
|
Brand name |
Ethyol (MedImmune) [USAN previously used: Ethiofos.]. |
InChI:InChI=1/C5H15N2O3PS.3H2O/c6-2-1-3-7-4-5-12-11(8,9)10;;;/h7H,1-6H2,(H2,8,9,10);3*1H2
112901-68-5 Relevant articles
PROCESS FOR THE PREPARATION OF (ω -AMINOALKYLAMINO)ALKYL HALIDES AND CONVERSION TO AMIFOSTINE
-
Page/Page column 14-15, (2008/06/13)
The present invention relates to process...
AMIFOSTINE DIHYDRATE CRYSTALLINE COMPOSITION
-
Page/Page column 13-14, (2008/06/13)
The dihydrate of 2-(3-aminopropylamino)e...
NOVEL DIHYDRATE FORM OF AMIFOSTINE AND PROCESS FOR ITS PREPARATION
-
Page/Page column 16; 17; 18, (2008/06/13)
The present invention relates to a novel...
112901-68-5 Process route
-
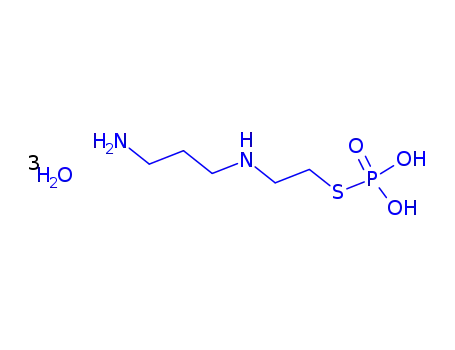
-
112901-68-5
amifostine trihydrate

-
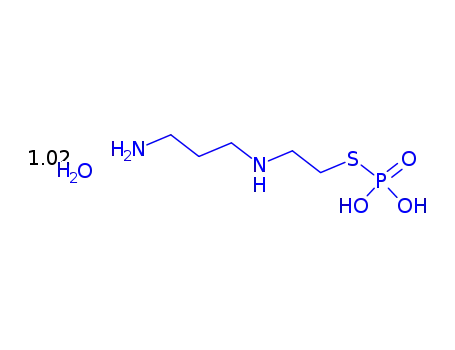
-
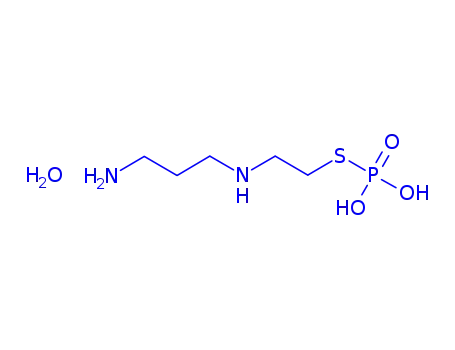
112901-68-5
amifostine monohydrate
| Conditions | Yield |
|---|---|
|
With
ion exchange resin; pyrographite;
In
methanol; water;
at -2 - 35 ℃;
|
-
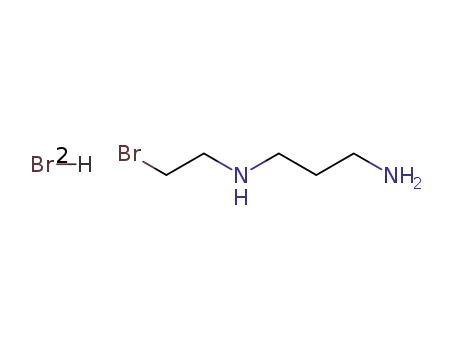
-
23545-42-8
dibromohydrate du N-(bromoethyl-2)diamino-1,3 propane

-
-
63717-27-1,112901-68-5
amifostine monohydrate
| Conditions | Yield |
|---|---|
|
With
water; trisodium thiophosphate;
N,N-dimethyl-formamide;
at 15 ℃;
for 1.5h;
|
112901-68-5 Upstream products
-
63717-27-1

amifostine monohydrate
-
23545-42-8
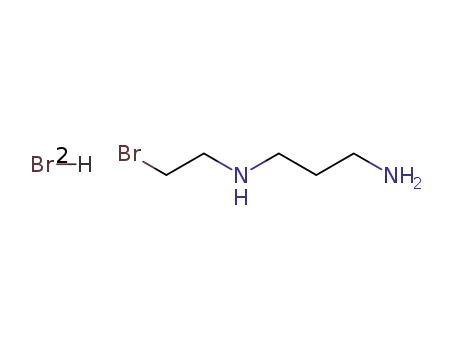
dibromohydrate du N-(bromoethyl-2)diamino-1,3 propane
Relevant Products
-
Sodium Ascorbyl Phosphate
CAS:66170-10-3
-
Terazosin hydrochloride dihydrate
CAS:70024-40-7
-
Isoniazid
CAS:54-85-3